Explain the Difference Between Osmosis and Diffusion in Cells
Movement or transportation in this process of osmosis tends to equalize the concentration of the solvent which doesnt occur. List the factors that influence permeability and the rate of diffusion Define hypotonic hypertonic isotonic and the effect they have on cells Analyze and explain the.

What S The Difference Between Diffusion And Osmosis
Osmosis is the movement of liquid solvent especially water from the higher region concentration to the lower region.

. Following are the substantial difference between osmosis and diffusion. If a red blood cell is placed in a solution that is 12 NaCl what if anything will happen to the blood cell. In both processes the particles go from a high concentration to a lower concentration.
Osmosis is the diffusion of solvent mostly water through a semipermeable membrane down its concentration gradient. The movement of materials always occur in cells. Plasmolysis of a cell when it is placed in a sugar or salt solution.
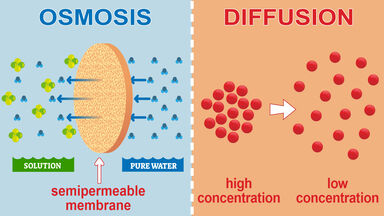
Diffusion Osmosis aka cell transport Lab Objectives. One big difference between osmosis and diffusion is that both solvent and solute particles are free to move in diffusion but in osmosis only the solvent molecules water molecules cross the membrane. Osmosis is a type of diffusion in which the solvent molecules move into the solution through a semi-permeable membraneExample.
Osmosis is the movement of water molecules but diffusion is for any molecule. The primary differentiating factor between the two systems is. Diffusion can occur with and without a membrane.
Include in your answer which type of the molecule is moving for each process in which direction the molecules in question are moving low to high or high to low concentrations. One big difference between osmosis and diffusion is that both solvent and solute particles are free to move in diffusion but in osmosis only the solvent molecules water molecules cross the membrane. Explain the major difference between diffusion and osmosis.
Explain the difference between osmosis and dialysis in your own words. Both osmosis and diffusion are very important for the survival of the cell. The differences between osmosis and diffusion it that diffusion refers to the movement of any chemical from one place to another whereas osmosis exclusively refers to.
In both diffusion and osmosis particles move from an area of higher. Diffusion is the movement of molecules such as oxygen in and out of a cell. Filtration is a process of transportation of substance passively between the compartments.
Osmosis is the net movement of water H_2O molecules from a region of less negative water potential to a region of more. The example of diffusion is the spreading of perfume in the air when it is sprayed while in osmosis the best example is the spread of water in the roots of plants. Explain the difference between osmosis and diffusion in cells.
Osmosis can only function in a liquid medium but diffusion can occur in all three mediums solid liquid and gas. In osmosis only water molecules pass through the cell membrane. Osmosis and diffusion are related processes that display similarities.
The movement of small molecules from a higher concentration to an area of lower concentration. This movement could occur by. The present post describes the Differences between Diffusion and Osmosis with a Comparison Table.
Define andor explain the following concepts. Diffusion is the process by which particles movie from high concentration gradient to lower concentration. The main difference between osmosis and diffusion is that osmosis requires a semi permeable membrane.
The diagram compares diffusion of sugar molecules and osmosis. Use the concept of osmosis to explain what would happen if you drunk salt water Preliminary Exercises - Osmosis and Diffusion Name. The process by which water molecules are able to diffuse through the cell membrane.
Both diffusion and osmosis are two vital passive transport that facilitates the movement of molecules in and out of the cell. In both diffusion and osmosis the phenomenon of particle transport occurs due to the difference in concentrations in different areas of the container that contains them and it is always in the. In biology this is a difference between the two processes.
Furthermore osmosis requires a semi-permeable membrane while diffusion does not. Osmosis is a special version of diffusion in which water molecules move from a higher water potential to a lower water potential across a semipermeable membrane. Osmosis is the movement of water molecules through the cell.
Osmosis depends upon the number of the solute particles which are dissolved into the solvent. Click to see full answer. Diffusion osmosis solute solvent solution kinetic theory of matter and selectively permeable.
Diffusion is the net movement of a substance from a region of higher concentration to a region of lower concentration down a concentration gradient. Substances move from a high to a low concentration down a concentration gradient. In chemistry its possible for other solvents to be involved.
Movement or transportation in diffusion tends to equalize the concentration throughout. Both osmosis and diffusion equalize the concentration of two solutions. The primary differentiating factor between the two systems is the medium in which they are employed.
During osmosis cells undergo different states which reflect the net movement of water molecules. Carbon dioxide oxygen water food substances wastes. In Osmosis the particles of solvent molecules travel across the semipermeable membrane while in diffusion the molecules from a higher to lower concentration.
The difference between osmosis and diffusion is that diffusion does not involve a semi permeable membrane. Lets go with diffusion and osmosis. The motion of molecules is through the semi-permeable membrane in osmosis whereas in diffusion motion is.
This is diffusioncould happen in liquids or gases. The lesson provides the core difference between diffusion and osmosis in table form for easier understanding during revision for competitive exams. Both diffusion and osmosis are passive transport processes which means they do not require any input of extra energy to occur.
Osmosis happens across a partially permeable membrane while diffusion does not need a membrane it happens directly in the fluid.

Major Difference Between Osmosis And Diffusion Similarities Yb Study
No comments for "Explain the Difference Between Osmosis and Diffusion in Cells"
Post a Comment